ELECTROPHILIC ADDITION
TO UNSYMMETRICAL ALKENES
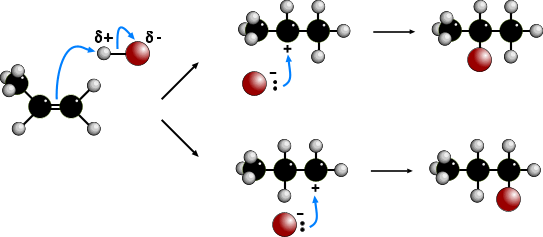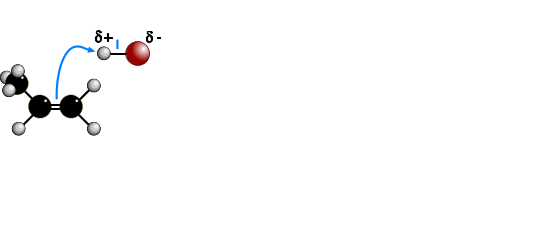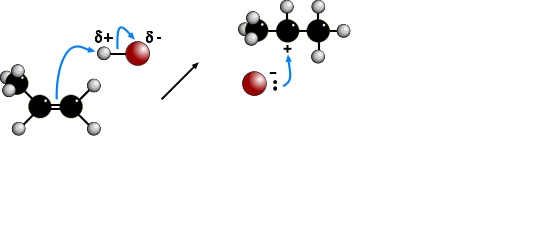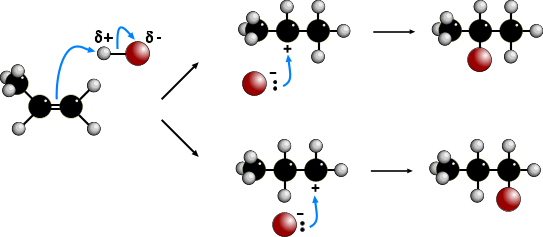 The electrophilic addition of hydrogen halides to UNSYMMETRICAL alkenes produces
two products because of the two different possibilities of adding the halide.
According to MARKOVNIKOV'S RULE, the reaction proceeds via the more stable
carbocation (carbonium) ion. In the animation the first route is via a SECONDARY
carbocation and the second route is via a PRIMARY carbocation. Secondary carbocations
are more stable than primary carbocations so are formed more readily. Once formed, both
carbocations react very quickly. The preferred product will be 2-bromopropane.
The electrophilic addition of hydrogen halides to UNSYMMETRICAL alkenes produces
two products because of the two different possibilities of adding the halide.
According to MARKOVNIKOV'S RULE, the reaction proceeds via the more stable
carbocation (carbonium) ion. In the animation the first route is via a SECONDARY
carbocation and the second route is via a PRIMARY carbocation. Secondary carbocations
are more stable than primary carbocations so are formed more readily. Once formed, both
carbocations react very quickly. The preferred product will be 2-bromopropane.
 To repeat, click on the
REFRESH icon
To repeat, click on the
REFRESH icon
 The electrophilic addition of hydrogen halides to UNSYMMETRICAL alkenes produces
two products because of the two different possibilities of adding the halide.
According to MARKOVNIKOV'S RULE, the reaction proceeds via the more stable
carbocation (carbonium) ion. In the animation the first route is via a SECONDARY
carbocation and the second route is via a PRIMARY carbocation. Secondary carbocations
are more stable than primary carbocations so are formed more readily. Once formed, both
carbocations react very quickly. The preferred product will be 2-bromopropane.
The electrophilic addition of hydrogen halides to UNSYMMETRICAL alkenes produces
two products because of the two different possibilities of adding the halide.
According to MARKOVNIKOV'S RULE, the reaction proceeds via the more stable
carbocation (carbonium) ion. In the animation the first route is via a SECONDARY
carbocation and the second route is via a PRIMARY carbocation. Secondary carbocations
are more stable than primary carbocations so are formed more readily. Once formed, both
carbocations react very quickly. The preferred product will be 2-bromopropane.
 To repeat, click on the
REFRESH icon
To repeat, click on the
REFRESH icon